mRNA·LNP Platform Technology
🧬 mRNA Platform Stability Simulator
Comparison of RNase resistance: SML's Circular Poly A vs. Conventional Linear Poly A
An innovative mRNA overcoming conventional mRNA limitations
Higher expression levels
Achieves higher expression rates both in vitro and in vivo, enabling greater protein production with lower mRNA quantities.
Enhanced stability
Improved in vivo stability prolongs mRNA persistence in the body, partially addressing its inherent rapid degradation.
Optimized delivery
Proprietary LNP formulation ensures efficient intracellular delivery, maximizing therapeutic efficacy across diverse applications.
SML Biopharm possesses an mRNA platform with optimized UTR and poly(A) tail designed to enhance stability and expression efficiency. While advancing our next-generation mRNA platform and drug pipeline, we independently develop lipid nanoparticles (LNP) for efficient delivery, driving effective R&D and rapid research progress.
Immunotherapy
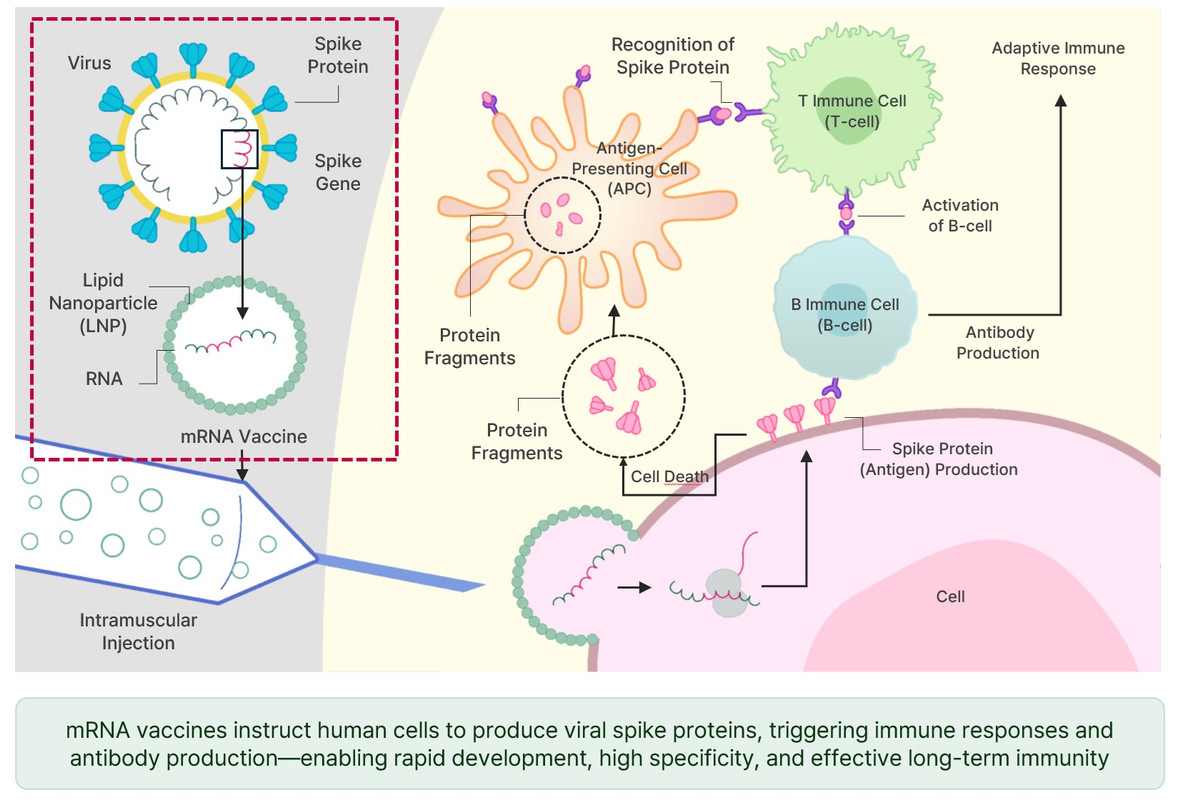
mRNA를 통해 특정 항원을 발현시켜 면역 반응을 유도하고 치료합니다. 예방 백신과 암백신을 포함한 다양한 신약 개발이 가능합니다.
In vivo Protein Therapeutics
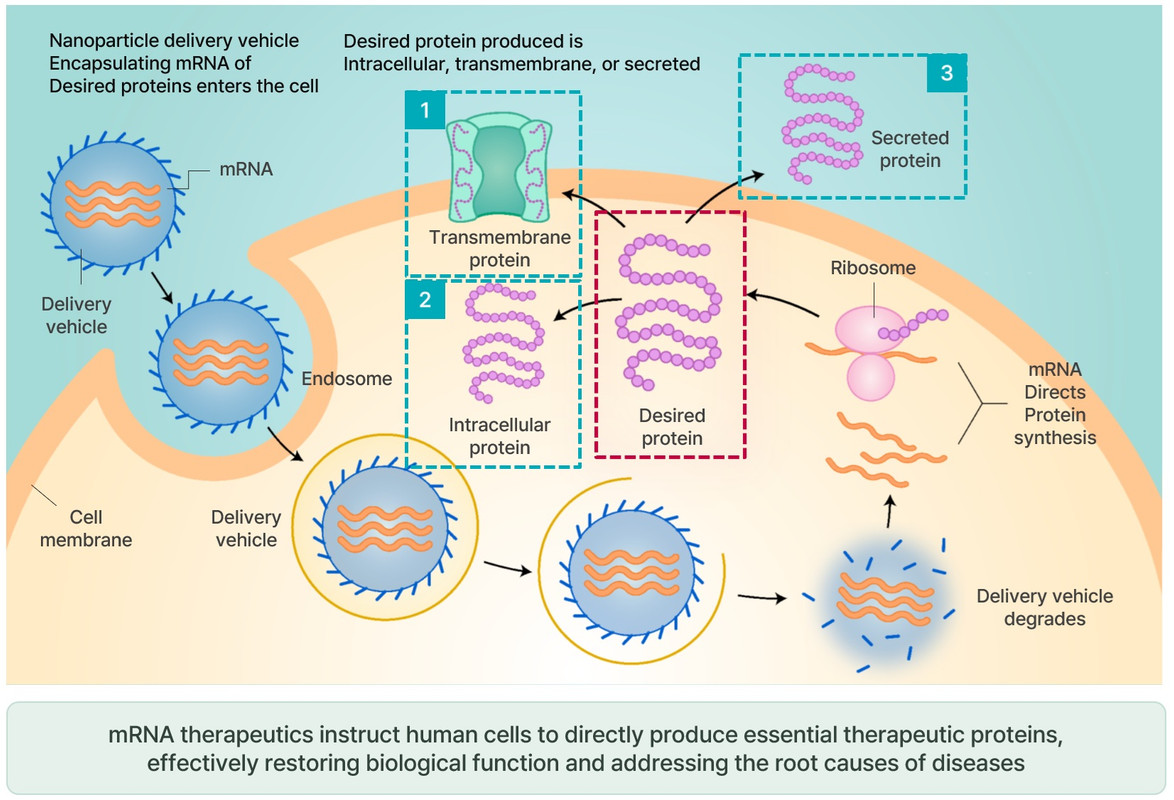
질병 치료를 위해 특정 펩타이드나 단백질(항체, 효소 등)을 발현시킵니다. 목표 단백질 생산이 훨씬 간단하고 효율적이며, 감염병, 암, 유전 질환, 희귀 질환 등 다양한 질환에 적용할 수 있습니다.
Pipeline
Developing innovative therapeutics for various diseases based on mRNA platform technology.
Cancer Vaccine
Immune Response Based Therapeutics
Optimization
Cancer Vaccine / mRNA-based bsAb
Immune Response Based Therapeutics
Optimization
Prophylactic Vaccine
Immune Response Based Therapeutics
Optimization
Antibody
In-vivo Protein Expression Therapeutics
Optimization
mRNA-based Protein (Novel Target)
In-vivo Protein Expression Therapeutics
Optimization
mRNA-based bsAb
In-vivo Protein Expression Therapeutics
Optimization
Development Process
SML Biopharm's drug development process
Discovery
Target discovery and
candidate exploration
Preclinical
Preclinical testing and
efficacy/safety validation
Clinical Trial
Clinical trials
(Phase 1-3)
Regulatory
Regulatory submission
and approval
Commercialization
Commercialization and
market launch
Patents
SML Biopharm's patent portfolio
